The Act on the Registration and Evaluation of Chemicals (known as Korea REACH) passed the plenary session of the National Assembly in Korea on April 30, 2013 and has come into force on Jan 1, 2015. Dec. 2016, the Ministry of Environment (MoE) started to revise the K-REACH and the amended K-REACH came into force from 1 Jan. 2019.
The purpose of K-REACH is to protect public health and the environment through these provisions:
- Registration of chemical Substances;
- Screening of hazardous chemical substances;
- Hazard and risk assessment of products containing chemical substances and hazardous substances;
- Sharing information of chemical substance.
The Ministry of Environment (MoE) is responsible for the registration and evaluation of chemical substance under this Act.
According to the content of Korea REACH, we summarized some key dates as follows:
|
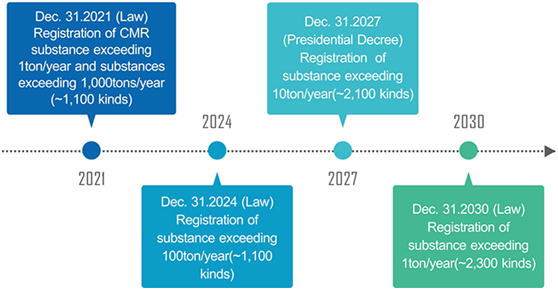
31 December 2030 |
Deadline for registration of substances exceeding 1t/y. |
|
31 December 2027 |
Deadline for registration of substances exceeding 10t/y |
|
31 December 2024 |
Deadline for registration of substances exceeding 100t/y |
|
31 December 2021 |
Deadline for registration of CMR substances exceeding 1t/y and substances exceeding 1000t/y |
|
30 June 2019 |
Deadline for K-REACH pre-registration |
|
1 January 2019 |
Commencement of the amended K-REACH |
|
1 July 2018 |
End of grace period - PECs joint registration |
|
21 November 2015 |
K-REACH: new substances notification leniency programme ends |
|
1st, July, 2015 |
Release the 1st List of existing substances subject to registration under K-REACH |
|
30 June 2015 |
Inform MoE of existing (TCCA) new substance hazard assessment results |
|
1 January 2015 |
"K-REACH" comes into force |
|
31 March 2014 |
Submission deadline: existing chemicals hazard and risk assessment-TCCA |
Scope of K-REACH
Korea REACH does not apply to:
- Radioactive substances of the Atomic Energy Act;
- Pharmaceuticals and non-pharmaceutical drugs of the Pharmaceutical Affairs Act;
- Narcotics of the Act on the Control of Narcotics;
- Cosmetics and materials of the Cosmetics Act;
- Ingredients and agrochemicals of the Agrochemicals Control Act;
- Fertilizers of the Fertilizer Control Act;
- Food, food additives, tools, containers and package of the Food Sanitization Act;
- Livestock feeds of the Act on Control of Livestock and Fish Feeds;
- Ammunitions of the Act on the Control of Firearms, Swords, Explosives;
- Military supplies of the Act on the Management of Military Supplies and of the Defense Acquisition Program Act (except for commercial goods under Article 3 of the Act on the Management of Military Supplies;
- Health functional food of the Health Functional Food Act;
- Medical devices of the Medical Device Act.
- Personal care products of Act on the Safety of Personal Care Products.
- Biocidal products of Consumer Chemicals and Biocide Safety Act.
Overview of K-REACH
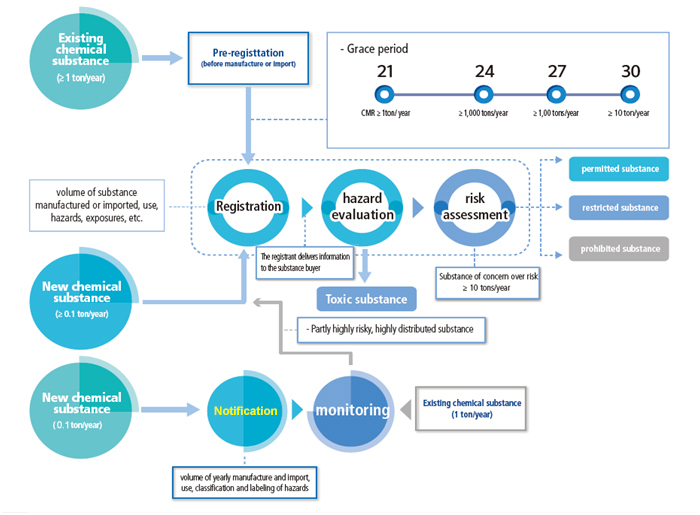
Pre-registration
In accordance with the requirements of the amended K-REACH, manufacturers/importers that manufactured or imported at least one ton per year of an existing substance between 2016 and 2018, shall submit application for pre-registration between 1 Jan. 2019 and 30 Jun. 2019. Enterprises that have completed the pre-registration can enjoy a corresponding registration grace period based on the tonnage band as well as the hazards of substances.
Enterprises that have not completed pre-registration or registration cannot continue to manufacture/import/export substances exceeding 1 ton/y after 1 Jul. 2019. If related enterprises illegally manufacture/import/use substances, the responsible enterprise or responsible person may be imposed imprisonment of no more than 5 years or a fine not exceeding 100,000,000 won, depending on the number of illegal activities and the seriousness of consequences.
Please click to view the List of CMR Substances
Registration
Enterprises that intend to manufacture or import the following substances are obligated to complete K-REACH registration:
- At least 0.1 ton/y of a new chemical substance;
- Existing substances manufactured, imported or sold more than 1 ton per year;
New substances manufactured or imported at least 0.1 ton/y shall be registered prior to manufacturing or importation. A grace period (given to pre-registered substances) will be granted to existing substances to be manufactured/ imported at least 1 ton/y.
Similar to EU REACH, foreign manufacturers exporting chemical substances or products containing hazardous chemical substance into South Korea may appoint an Only Representative to fulfill relevant obligations under K-REACH.
Definition of New Substances
A new substance is defined as a substance that is not on the following lists:
- Chemical substances which were placed on Korean market before Feb. 2, 1991, and notified by the Ministry of Environment on Dec. 23, 1996; and
- Chemical substances which have undergone the examination of toxicity under the former provisions or the provisions of this Act after February 2, 1991 and were announced by the Minister of Environment.
Please click here to search Korea Existing Chemicals Inventory (KECI).
Information Required for Registration
The following information is required for registration:
- The name, address and representative of a manufacturer or an importer or an only representative;
- Information that identifies a chemical substance including its name, molecular formula and graphic formula;
- Identified uses of the chemical substance;
- Classification and labeling of the chemical substance;
- Physical and chemical properties;
- Hazard data (tox./eco-tox. data);
- Risk associated with the chemical substance including exposure scenarios describing how to handle and control it (Applicable only when the substance is manufactured or imported in 10 tons or more per year);
- Guidance on safe uses(including protective equipments, response to an explosion, a fire or a leak);
- Other information specified in the Environment Ministerial Decree
Registration - Joint Submission
Enterprises that intend to register existing chemical substances within the registration grace period shall individually apply for registration, but in cases of the data for application for registration such as test data for hazards of chemical substances, they shall jointly submit such data by forming consortium. The procedures of joint registration can be concluded as follows:

Registration – Polymer Registration
Polymer registration requires less data on the properties of the polymer, e.g. acid/base stability test, and residual monomer contents test than normal (non-polymer) registration.
In accordance with the paragraph 3 article 2 of K-REACH Enforcement decree, a polymer is defined as a substance meeting the following criteria:
- It shall be composed of molecules in which at least one kind of monomer unit is continuously repeated;
- It shall show the characteristic distribution of molecular weights in accordance with repetitive numbers of monomer units in each molecule;
- Its molecules that at least three monomer units form a covalent bond with at least one monomer unit or other reactants shall be at least 50 percent; and
- Its molecules of the same molecular weight shall be not more than 50 percent of the weight ratio.
Chemical Substances Exempted from Registration or Notification
Substances falling under any of the following subparagraphs are subject to exemption from registration:
1. A chemical substance imported as contained in machinery;
2. A chemical substance imported along with machinery or equipment for a test run;
3. A chemical substance in a product in solid state with specific shape for a certain function and not discharged during its use;
4. A chemical substance designated and publicly notified by the Minister of Environment after deliberation by the Chemical Substance Evaluation Committee which has extremely low risk;
5. Impurities, by-products, minerals, ores, glass, vegetable fats/oil, hydrogen, and oxygen, etc.;
6. A substance itself existing in the nature or a substance obtained by using manpower, machine or gravity to the substance existing in the nature or by selecting after dissolving or floating thereof in the water; and
7. A base composing DNA or RNA, nucleoside which is a combination of base and sugar.
The following substances will also be exempted from registration or notification after obtaining confirmation of exemption from registration. Application for confirmation of exemption from registration shall be made via the Chemical Substance Information Processing System before manufacturing or importing:
- Reagents for test, research or inspection; or
- A chemical substance for demo production, such application may be submitted within 30 days from the date such substance has been manufactured or imported (within 14 days until Dec. 31, 2018).
Supply Chain Communication
Anyone who transfers a registered chemical substance or preparation containing the substance shall provide the following information to downstream users:
- Name or trade name, location and phone number of the provider of the chemical substance safety information;
- Name of the product and name or generic name of the relevant chemical substance;
- Registration number or report number and serial number of the relevant chemical substance
- Classification and labeling of the relevant chemical substance;
- The uses available for use or the uses restricted for use of the relevant chemical substance;
- Physical and chemical properties of the relevant chemical substance and the information relating to human bodies and environmental hazards;
- Summarized information of the exposure scenario and the risk information such as measures to reduce the risks;
- Summarized information of the exposure scenario and the risk information such as measures to reduce the risks;
- Information concerning safe use of the relevant chemical substance such as operational methods of the relevant chemical substance, measures in emergency such as fire, manner of control at the time of leaking, protective equipment and method of disposal; and
- Regulatory information for the relevant chemical substance;
Product Management under K-REACH
Notification of products
Enterprises that manufacture or import any product containing any substance of priority control shall notify the Minister of Environment before manufacturing or importing if the product corresponds to both of the following requirements:
1) The content of the individual substance of priority control per one product exceeds 0.1 weight percent; and
2) The gross weight by substance of the substances of priority control contained in the total products exceeds one ton per year.
Substances falling under any of the following are substances of priority control:
- A substance which may be or are concerned to be carcinogenic, mutagenic, toxic for reproduction, or disruptive to endocrine system to human beings or animals;
- A substance which is easily accumulated in the bodies of human beings, animals or plants and remains in the environment for a long period of time;
- A substance which may damage organs such as lungs, livers and kidneys of human beings if human beings are exposed for a long period of time or repeatedly to such substance; and
- A substance which may give the same level or higher level of serious risk to human beings, animals or plants compared with the above substances.
Please click to view the List of Priority Control Substance
Our Korea Chemical Compliance Services
- K-REACH Only Representative
- K-REACH Pre-registration
- K-REACH Registration
- K-REACH Lead Registrant Service
- New Substance Inquiry
- K-REACH Registration Exemption Application
- Risk Assessment Report
- Technical Support
- Preparation of Korean SDS and label;





